|
MAIN PAGE
> Back to contents
Law and Politics
Reference:
Belikova K.M.
The force of patent law in Brazil applicable to pharmaceutical products: legal issues and ways for their solution prior and after Brazil’s membership in WTO
// Law and Politics.
2019. № 7.
P. 1-12.
DOI: 10.7256/2454-0706.2019.7.29922 URL: https://en.nbpublish.com/library_read_article.php?id=29922
The force of patent law in Brazil applicable to pharmaceutical products: legal issues and ways for their solution prior and after Brazil’s membership in WTO
Belikova Ksenia Michailovna
ORCID: 0000-0001-8068-1616
Doctor of Law
Professor of the Department of Entrepreneurial and Corporate Law, Kutafin Moscow State Law University, Professor
125993, Russia, Moscow, Sadovaya-Kudrinskaya str., 9

|
BelikovaKsenia@yandex.ru
|
|
 |
Other publications by this author
|
|
|
DOI: 10.7256/2454-0706.2019.7.29922
Received:
03-06-2019
Published:
16-07-2019
Abstract:
This article examines the questions of the force of patent law in one of the BRICS countries – Brazil, with regards to pharmaceutical products prior and after Brazil’s membership in WTO (TRIPS) in light of a number of regulatory and judicial acts and technicality in the area of healthcare, provision of population with medications, and protection of industrial property rights (Constitution of 1988, Law No. 9.279 of May 14, 1996 “On the Industrial Property”, Law No. 8.080/90 on creation of the Unified Healthcare System (SUS), Ordinance No. 3.916/98 establishing National policy on Pharmaceuticals, Government Decree No. 2.577/06 “National Program for Exceptional Medicines”, court decisions, and others). The research analyzes the issues towards free access to medications, prohibited in Brazil by patent law, since its entry to World Trade Organization, as well as the ways for their solution. The scientific novelty consists in the comprehensive analysis from the perspective of the intellectual property right of Brazil’s approaches to organizational-legal support of the development of pharmaceutical sector in the context of TRIPS agreements and necessity to ensure population’s constitutional right to health services and essential medicines. The conclusion is made that the current policy is aimed at achieving the existing prior to WTO membership balance of private and public interests via implementation of a set of compensation mechanisms (negotiations on price reduction by pharmaceutical companies, obligatory licensing, introduction of the Program “National Pharmacology of Brazil”.
Keywords:
BRICS, Brazil, health care, pharmacology, pharmaceutical companies, patents, patented drugs, TRIPS, Popular Pharmacy, compulsory licensing
This article written in Russian. You can find original text of the article here
.
За последние несколько десятилетий фармакология, как и медицина, сделала существенный шаг в своем развитии. Появились новые, в том числе эффективные препараты, на которые не перестают рассчитывать пациенты, и спрос на них только увеличивается. Поэтому представляется необходимым сделать предметом внимания некоторые аспекты защиты фармацевтических продуктов действующим патентным правом. Для исследования выбрано право одной из стран БРИКС – Бразилии, как имеющей в этой сфере определенные достижения, так и столкнувшейся после вступления в 1995 г. во Всемирную торговую организацию (далее – ВТО) с проблемами правового характера.
После вступления в ВТО в Бразилии произошел резкий сдвиг в политике фармацевтического производства. Ранее бразильское законодательство (п. с) ст. 9 Закона 5.772/71, утвердившего Кодекс промышленной собственности 1971 г. [1]) запрещало патентование фармацевтических продуктов и процессов. Это позволяло правительству рассчитывать на сравнительно дешевые отечественные дженерики для удовлетворения своих потребностей в сфере здравоохранения. Однако, на основании ст. 27 Соглашения по торговым аспектам прав интеллектуальной собственности (ТРИПС) [2], заключенного Бразилией-участницей ВТО, она обязательно должна выдавать патенты на лекарства. Это обусловлено тем, что предписание п. 2 ст. 27 разрешает членам ВТО запрещать патентование изобретений, способных при коммерческом использовании в пределах их территорий нарушить на них общественный порядок или мораль, включая жизнь или здоровье живых существ (людей, животных, растений), или привести к серьезному ущербу окружающей среде. При этом такое исключение недопустимо, если оно используется вследствие запрета национального законодательства.
Для того, чтобы удовлетворить этим и другим требованиям в Бразилии в 1996 г. был принят и в 1997 г. вступил в силу новый Закон № 9.279 от 14 мая 1996 г. «О промышленной собственности» [3] (в ред. Закона № 10.196 от 2001 г., далее – Закон 1996 г.), который с 15 мая 1997 г. является основным патентным законом страны [4. P. 1-10]. Соблюдение положений ТРИПС, естественно, сказалось на стоимости лекарственных средств, поскольку исключило возможность производства дженериков в течение срока действия патента на фармацевтическую продукцию. Это вредоносное воздействие усугубилось и тем фактом, что Бразилия не смогла воспользоваться рядом гибких возможностей, которые ограничивали бы патенты на фармацевтические препараты и помогли бы сохранить регулируемость цен на лекарства (напр., страна отказалась от параллельного импорта лекарств и др., об этом ниже).
По вступлении Бразилии в ВТО в 1995 г. и до момента вступления в силу на основании Закона 1996 г. патентной реформы ряд компаний подал иски, требуя немедленного признания патентоспособности некоторых лекарственных препаратов. В то время перед учеными-юристами встал вопрос о том, должны ли положения ТРИПС в связи со сложившейся ситуацией применяться судами до принятия нового патентного законодательства. Некоторые бразильские ученые выступали за немедленное применение положений ТРИПС, другие оправдывали применимость постепенного подхода [5. P. 3-14; 6]. Однако суды (напр., Федеральный региональный трибунал Второго региона [7] и др. Подробнее о судебной системе и правоприменении по делам из нарушений прав интеллектуальной собственности см. [8; 9]) последовательно выносили решения в пользу немедленной патентной защиты. Это означало, что еще до принятия законодательным органом Закона 1996 г. положения ТРИПС, касающиеся фармацевтических продуктов, были введены в действие в судебном порядке. Такое решение работало, как и везде, где принимаются подобные решения, на благо отрасли, но против интересов общественного здравоохранения, которых, в качестве аргументов, большинство таких пропатентных судебных решений не касалось.
Чтобы понять этот вопрос лучше, необходимо коснуться его предыстории [15. P. 165-197]. Доступ к лекарственным средствам в Бразилии следует рассматривать в более широком контексте национальных правовых предписаний, касающихся права на здоровье. Согласно ст. 196 Конституции 1988 г. [10] каждый имеет право на сохранение здоровья, а обязанностью государства является гарантировать это право проведением соответствующей социально-экономической политики, нацеленной на снижение заболеваемости и связанных с ней опасностей за счет введения мероприятий по всеобщему (universal) и равному (equal) доступу к услугам, обеспечивающим поддержание, защиту и восстановление здоровья.
При этом в развивающем это конституционное положение законодательстве, как и в самой Конституции, в которой здоровье относится к числу основных прав человека, это предписание имеет характер не цели-принципа, но положения немедленного и прямого действия. Развивающее эти конституционные предписания законодательство состоит из:
· Закона 8.080/90 (Lei 8.142/90 (D.O.U. de 31.12.1990. URL: conselho.saude.gov.br/web_confmundial/docs/l8142.pdf (дата обращения: 17.05.2019)), на основе которого создана и действует Единая служба здравоохранения (Sistema Único de Saúde, SUS), которая гарантирует охват медицинским обслуживанием всех граждан Бразилии, обеспечивая доступ к нему порядка 60 млн. чел. (До 1988 г. медицинская помощь Национального института медицинской помощи и социального обеспечения (INAMPS), была доступна только обеспеченным слоям населения, остальные рассматривались как «неимущие» и получали помощь только как благотворительность. [11]) Согласно ст. 6 (I) (d) этого Закона SUS ответственна за оказание населению полной медицинской помощи, которая включает в себя и фармацевтическую помощь. Согласно этому Закону SUS основывается на трех основополагающих принципах. Во-первых, система здравоохранения универсальна, т.е. ни один гражданин не может быть исключен из покрытия SUS (ОМС в смысле российского права (для оценки подхода Бразилии в этом вопросе полезно ознакомиться и с подходами в смежных политиках [12; 13. С. 44-55; 14. С. 83-95]). Здесь нужно отметить, что, несмотря на растущий рынок услуг добровольного медицинского страхования, по данным Национального совета секретарей здоровья (Conselho Nacional de Secretários de Saúde) - негосударственной организации, цель которой - обсуждение общих интересов Секретариатов здравоохранения штатов (Secretaria de Saúde, каждый штат имеет свой Секретариат, орган подчиняется губернатору штата), например, на 2006 г. 90 % населения пользовалось SUS [16]. Во-вторых, она нацелена на равные возможности (без дискриминации) пользования услугами и продуктами, предоставляемыми государственным здравоохранением. В-третьих, она предполагает реализацию «полного покрытия» при оказании медицинских услуг, то есть, от самых основных базовых простых распространенных до сложных и редких (есть исключения, конечно) потребностей в области здравоохранения. В настоящее время этот принцип «полного покрытия», по нашему мнению, является (в связи с действующими с момента присоединения в ВТО правилами относительно патентования фармпродукции, начиная с молекулы) основным принципом и, даже скорее, подходом, который (как станет видно дальше) создает проблемы. Итак, согласно Закону 8.080/90 право на доступ к медицинским препаратам рассматривается как составная часть права на доступ к здравоохранению. Как следствие этого возникла необходимость в актах этой сферы, которые мы приведем ниже;
· Декрета 3.916/98 (Portaria 3.916/98 (URL: http://bvsms.saude.gov.br/bvs/saudelegis/gm/1998/prt3916_30_10_1998.html (дата обращения: 17.05.2019), которым была утверждена Национальная политика в области лекарственных средств (Política nacional de medicamentos), направленная на обеспечение доступа к лекарствам по доступным ценам, а также их безопасности, эффективности и качества. Согласно этой Политике Министерству здравоохранения было поручено подготовить перечень основных лекарственных средств (Relação Nacional de Medicamentos Essenciais ((RENAME). Он был подготовлен и периодически обновляется Министерством здравоохранения в соответствии с критериями эффективности лекарственных препаратов (появляются новые, к старым бактерии и вирусы становятся резистентными и пр.), безопасности и стоимости лекарств, доступных на рынке. Список включает в себя все препараты, необходимые для лечения и профилактики основных заболеваний, распространенных в стране. Препараты, необходимые для дорогостоящего лечения менее распространенных состояний (напр., хроническая почечная недостаточность, трансплантация органов и тканей и пр.) – не входят в этот список. Поскольку Конституцией гарантируется оказание помощи как тем, кто страдает от самых распространенных заболеваний, так и тем, кто страдает заболеваниями, которые затрагивают лишь ограниченную часть популяции в стране, и лечение которых является (как правило) дорогостоящим, был принят еще один акт в этой сфере;
· Постановления Правительства 2.577/06 (Ordinance 2.577/06), которым была утверждена Национальная программа редких лекарственных средств (National Program for Exceptional Medicines) для обеспечения поставок стратегических медикаментов для лечения ВИЧ/СПИДа, малярии, туберкулеза, гриппа и менингита [25].
В дополнение к этим актам общего характера были приняты некоторые законы, касающиеся конкретных заболеваний или групп населения:
· Закон 8.069/90 предусматривает, что дети и подростки имеют право на полное покрытие государством медицинского обслуживания на любые цели.
· Закон 9.313/96 обязывает SUS предоставлять бесплатные лекарства всем гражданам, инфицированным ВИЧ/СПИДом,
· Закон 10.741/03 требует, чтобы SUS предоставляла полную медицинскую помощь пожилым людям, и особое внимание уделяет их особенным потребностям в области здравоохранения (напр., необходимости ухода за ними и их содержания в специализированных клиниках и др.),
· Закон 11.347/06 гарантирует бесплатные лекарства всем пациентам с диабетом (URL: bvsms.saude.gov.br (дата обращения: 18.05.2019)).
Как же такое возможно? Вот тут и сталкиваются два практических подхода, - первый, который до определенного времени (до 2007 г.) безоговорочно поддерживался бразильскими судами, о том, что личные права пациентов на здоровье и на медицинские препараты должны быть удовлетворены несмотря ни на какие финансовые затраты государства, второй (более позднего времени, с 2007 г.), что, гарантируя права личности, необходимо учитывать публичный интерес, то есть интерес государства и остальных пациентов, которые не требуют таких препаратов в суде, но которые тоже нуждаются в иной медицинской помощи.
Эта ситуация сложилась из-за того, что на практике случалось, что лекарства из названных выше списков иногда пациентам не предоставляются. В этом случае отдельные лица могут обращаться в суд за принуждением к реализации их права доступа к лекарственным препаратам. Такие иски обычно касаются вопроса предоставления лекарств, которые: 1) включены в один из списков поставок Министерства здравоохранения, но по ряду причин отсутствуют; 2) не включены в списки поставок из-за соображений стоимости, в том числе тех, для которых имеются более дешевые дженерики; и 3) еще не были признаны безопасными и эффективными в ходе национальных клинических исследований.
По общему правилу первоначально и долгое время бразильские суды выносили решения в пользу истцов, требовавших лекарств. Судебный консенсус состоял в том, что каждый раз, когда правительство отказывает в лекарстве, оно нарушает основополагающее конституционное право. Исследование бразильского прецедентного права показывает, что большинство судей: 1) рассматривало право на здоровье как право индивидуальное, личное, принадлежащее каждому отдельному пациенту в каждом отдельно взятом случае, а не как право коллективное, принадлежащее группе лиц (т.е., с позиции распределения затрат на отдельного пациента из расчета общей суммы государственного финансирования, выделенного на всю данную группу лиц, к которой принадлежит пациент – истец); 2) толковали право на здоровье и принципы SUS как право человека на любые расходы, связанные со здоровьем (неважно дорогое это лекарство или дешевое); 3) игнорировали тот факт, что осуществление таких прав сопряжено со значительными расходами, что потребности системы здравоохранения в целом огромны и что нехватка государственных средств обусловливает необходимость бюджетных компромиссов; и 4) не принимали во внимание уже существующую политику распределения лекарств. Такие решения принимали и Трибуналы юстиции Штатов, и Высший трибунал юстиции, и Федеральный верховный трибунал.
Исключение составляли случаи лечения за границей, что мотивировалось такими обоснованиями, которые затем стали применяться судами и к искам пациентов внутри страны. Это идеи о том, что: 1) оплата такого лечения создаст большие трудности для государственных органов и сделает систему здравоохранения практически недействующей (все средства могут быть исчерпаны небольшим числом обращений); 2) нельзя игнорировать нехватку ресурсов; и 3) следует соблюдать технические и административные критерии, выбранные государством в качестве приоритетов политики в области здравоохранения, с тем чтобы содействовать более рациональному расходованию государственных средств и приносить пользу как можно большему числу граждан. Два первых решения об отказе в предоставлении медицинских препаратов были вынесены в 2007 г. Федеральным верховным трибуналом. Они вызвали бурную протестную реакцию гражданских активистов и пациентов, в том числе в СМИ, тогда как местные чиновники системы здравоохранения тут же подали свои иски о прекращении медицинского обслуживания в форме выдачи бесплатных медицинских препаратов, что было ранее предписано судами низшей инстанции. Последующие решения, принимаемые Федеральным верховным трибуналом, это попытка умиротворить обе стороны: часть решений поддерживала прежний подход, часть отражала нынешний. Если подумать неэмоционально, действительно, удовлетворение всех подряд исков, из которых, как нами уже отмечалось ранее [9], выигрываются те, в которых истец нанимает хорошего частного адвоката, услуги которого стоят дорого (т.е. истец заведомо имеет деньги, но просит бесплатные лекарства, значит это способствует поддержке неравенства, ибо бедный пациент не может нанять дорогого адвоката и не может потому получать лекарства и т.д. по кругу, следовательно, исковые средства защиты – не для всех, а это опять неравенство), это большое бремя для государства. Чтобы не быть несправедливым и не рассуждать без оснований, государство провело ряд исследований того, кто реально получает выгоду от выигрыша таких дел. Выяснилось также, что большинство людей, которые обратились в суд, имели медицинские рецепты от частных врачей, а те, из обратившихся, у кого были самые высокие доходы, требовали самых дорогих лекарств бесплатно [17. P. 40-43]. Поэтому, видимо, в плане немедленного применения положений ТРИПС о патентовании медицинских препаратов, бразильские власти решили, что те, кто не сможет покупать лекарств по ценам, которые взлетят по причине, указанной нами выше, после начала применения новых правил, не могли это делать и на рассматриваемый период времени, а те, кто требовал лекарств бесплатно, вполне может позволить их себе за деньги.
Возвращаясь в этом формате к мерам, которые ухудшили состояние фармацевтического рынка и право доступа к лекарственным препаратам, рассмотрим введенные властями Бразилии запрет параллельного импорта и новый вид патентов, которые не предусматриваются ТРИПС, – так называемые pipeline patents.
Патентная реформа 1996 г. исключила возможность параллельного импорта, восприняв и проводя в жизнь принцип национального исчерпания права, что позволяет патентообладателям предотвращать импорт их продукции в Бразилию неавторизованными поставщиками [18. С. 36-44]. На практике это означает, что фармацевтические компании могут устанавливать более высокие цены на лекарства в Бразилии, когда этот же препарат законно продается по более низкой цене где-либо еще в мире.
Эта ситуация послужила основанием инициативы конгрессмена от Бразильской социал-демократической партии Альберто Голдмана (Законопроект №139 1999 г.) о разрешении параллельного импорта, в том числе медикаментов [19]. Однако, до сих пор эта идея не нашла одобрения.
Что касается вопроса выдачи т.н. pipeline patents, то Закон 1996 г. в ст. 230 и 231 позволяет подавать патентные заявки на ранее не подлежавшие патентованию (по причине изъятий, например) объекты в максимально упрощенном административном порядке при условии, что патент на такое изобретение уже был выдан за рубежом. Этим занимается в Бразилии с точки зрения формального анализа только административный орган - Национальный институт промышленной собственности, подчиненный министерству экономики (Instituto Nacional da Propriedade Industrial, INPI) (URL: http://www.inpi.gov.br/ (дата обращения: 10.05.2019)).
Поскольку п. 3 ст. 230 гласит, что после выполнения положений, установленных в ст. 230 Закона 1996 г., и подтверждения факта выдачи патента в стране, где была подана первоначальная заявка, патент выдается в Бразилии так же, как он был выдан в стране его происхождения.
При этом первая проблема состоит в том, что в стране происхождения патента требования к новизне, изобретательскому уровню или промышленной применимости может быть не таким высоким, как в Бразилии, поэтому у практиков и юристов возникли опасения относительно того, что некоторые лекарства, которые не могли бы быть запатентованы в Бразилии из-за высоких требований патентного закона, получили защиту за рубежом и потом попали-таки в Бразилию.
Вторая проблема заключается в том, что такой патент позволяет ретроспективно патентовать уже изобретенные и имеющиеся на рынке лекарства. Традиционный аргумент в пользу такой патентной защиты, состоящий в том, что высокие цены на те или иные товары (в нашем случае лекарства) нужны для стимулирования инноваций, не применим в этом случае, однако применим он или нет, компании, получающие такие патенты, в отношении лекарств, уже имеющихся на рынке, получают дополнительные финансовые выгоды от автоматически растущих цен на лекарства, не неся при этом каких-либо затрат по инвестированию в какие-либо дополнительные инновации.
Вместе с тем, такие патенты, как и любые другие, запрещают производство и покупку дженериков. Так были получены патенты на уже присутствующие на бразильском рынке лекарства для лечения ВИЧ/СПИДа (Лопинавир / Ритонавир, Абакавир, Нелфинавир, Ампренавир) и лейкемии (Гливек) и др., что резко повысило государственные расходы на поставку этих лекарств. В общем. такое патентование привело к патентной защите по меньшей мере 340 лекарственных препаратов и искусственно завысило цены на них, по причине отсутствия конкуренции со стороны производителей дженериков.
В связи с этой ситуацией в ноябре 2007 г. Национальная федерация фармацевтов (Federação Nacional de Farmacêuticos, FENAFAR) от имени Rede Brasileira pela Integração dos Povos, REBRIP (Бразильской сети интеграции народов (URL: http://www.rebrip.org.br/institucional/ (дата обращения: 28.05.2019))) - коллектива, объединяющего профсоюзы и иные профессиональные организации, общественные движения, женщин, защитников окружающей среды и др., которые хотят влиять на международную торговлю и интеграцию регионов – подала жалобу в Генеральную прокуратуру, заявив, что положения закона 1996 г. о такого рода патентах нарушают конституционные права граждан, а именно: 1) верховенство интересов общества и стремление к технологическому и экономическому развитию страны над защитой интеллектуальной собственности и 2) безусловные права общества на содержание таких патентов, поскольку защищаемые ими лекарства, уже были общественным достоянием. В апреле 2009 г. Генеральный прокурор Республики обратилась в различным частным и публичным организациям и ассоциациям (ABIFINA, Pró-genéricos, Conectas e GAPA/SP, ABIA, MSF, FENAFAR, IDEC, GIV e GAPA/RS, ANVISA, INTERFARMA, ABRASEM, ABPI) с просьбой выступить в качестве «друзей суда» (amicus curiae) [20] в судебном процессе по вопросу (не)конституционности таких патентов с тем, чтобы высказать свое мнение «за» или «против». Обзор мнений представлен в работе «Pipeline patents and access to drugs: economic and legal aspects deleterious to health economy» [21. P. 164-188]. Те, кто был «за» некоституционности положений ст. 230 и 231 Закона 1996 г. оказались в меньшинстве, тем не менее, Генеральная прокуратура обратилась в Федеральный верховный трибунал с требованием признания неконституционности этих положений Закона 1996 г., таким образом, судьба этого важного вопроса оказалась в руках судебной власти. Как нам удалось выяснить, на данный момент эти статьи пока действуют.
Таким образом, можно заключить, что с момента начала применения ТРИПС Бразилия предприняла ряд шагов, которые улучшают положение международных (иностранцы, ТНК) владельцев прав интеллектуальной собственности, но подрывают способность страны давать практическую реализацию конституционному праву на доступ к лекарствам.
Осознавая это, правительство стало реализовывать ряд инициатив, направленных на то, чтобы сделать лекарства более доступными. В их числе, например:
· Программа «Народная фармакология Бразилии» (Programa “FarmáciaPopulardoBrasil”), действующая на основе Декрета 5.090/04 (Decree 5.090/04) - принятая первоначально для улучшения доступа к основным лекарственным препаратам пациентов, которые используют частную систему здравоохранения, в виду того, что полисы добровольного медицинского страхования обычно не покрывают расходы пациентов на лекарства, на практике, она широко используется пациентами SUS, когда государственные больницы не в состоянии обеспечить пациентов лекарствами вовремя.
Программа работает как через государственные, так и через частные аптеки. Министерство здравоохранения закупает лекарства в частном и государственном секторах, а участвующие в Программе аптеки перепродают их по ценам до 90% ниже рыночных. Согласно правительственным данным [22], Программа: 1) позволяет нуждающимся в этом пациентам принимать лекарства постоянно, без перерывов, 2) смягчает воздействия необходимости покупки лекарств на семейные бюджеты; и 3) сокращает расходы на госпитализацию, вызываемую прерыванием лекарственного терапевтического лечения.
Тем не менее, в 2005 г. в штате Сан-Паулу был подан групповой иск, в котором оспаривалась возможность предоставления лекарств в рамках этой Программы за деньги, и утверждалось, что Программа нарушает конституционное право на здоровье, требуя от граждан платить за лекарства (пусть даже и по цене на 90 % ниже рыночной) вместо того, чтобы получать их бесплатно. Федеральный судья удовлетворил иск и обязал все субсидируемые государством аптеки предлагать населению лекарства бесплатно. Однако позже это решение было отменено вышестоящим судом, который счел эту Программу дополняющей услуги, оказываемые в рамках SUS, и потому не наносящей ущерба политике в области здравоохранения [23].
Что касается характеристики действия Программы, то рисунок 1 показывает рост числа участвующих в ней аптек с 2 тыс. 955 аптек до 25 тыс. 122; рост числа охваченных Программой муниципий, в которых действуют аккредитованные для работы в Программе подразделения аптечных сетей с 594 до 3 тыс. 730 муниципий и числа обслуживаемых такими аптеками в таких муниципиях пациентов с 476 тыс. 811 чел. до 12 млн. 953 тыс. 105 чел. в 2006-2012 гг.
Рис. 1. Рост количества аптек, муниципий, покрытых сетью таких аптек и числа пользующихся ими в рамках Программы “Farmácia Popular do Brasil” пациентов в 2006-2012 гг.
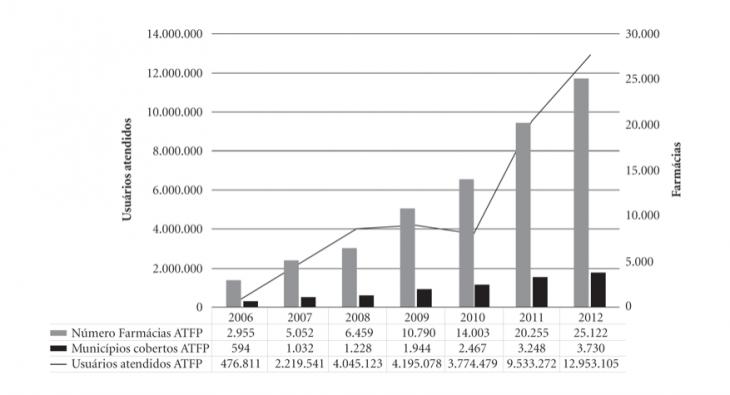
Источник: Rondineli Mendes da Silva, Rosangela Caetano. Op. cit.
· выдача принудительных лицензий и ведение переговоров о снижении цен на лекарственные препараты с ведущими фармацевтическими компаниями (главным образом, зарубежными и транснациональными). В качестве наглядного примера такой необходимости укажем, что до 1996 г. в бразильские суды были поданы сотни исков с требованием доступа к лекарствам от ВИЧ/СПИДа, иски удовлетворялись и работали в пользу всех: правительство не только предоставляло такие препараты отдельным пациентам, которые имели доступ к судам, но и ввело комплексную Национальную программу по лечению заболеваний, передающихся половым путем, и СПИДА (Programa Nacional de Doenças Sexualmente Transmissíveis e AIDS), чтобы сделать эти препараты легко доступными для всех.
Как отмечалось выше, еще до вступления в силу Закона 1996 г. рядом фармацевтических компаний были получены патенты на уже присутствующие на бразильском рынке лекарства для лечения ряда заболеваний (ВИЧ/СПИД, и лейкемия и др.), что резко повысило государственные расходы на поставку этих лекарств. Поскольку (наряду со сказанным выше по этому вопросу) бразильская сеть государственных фармацевтических лабораторий, сыгравшая, например, важную роль в эффективном осуществлении Национальной программы по лечению заболеваний, передающихся половым путем, и СПИДА, в плане создания эффективных лекарств против таких заболеваний застопорилась по причине того, что ранее она обеспечивала 18 противовирусных препаратов и препаратов по борьбе с сопутствующими таким заболеваниям и СПИДУ инфекциями, а затем зарубежные компании получили на эти препараты более 220 патентов в Бразилии, и теперь из 18 препаратов, поставляемых правительством, только 8 производятся бразильскими лабораториями.
Введение патентной защиты рассматриваемых фармацевтических препаратов повлекло рост цен на них, которые правительство все также в рамках этой Программы обязано закупать для тысяч пациентов у компаний-патентообладателей.
В этой ситуации cтолкнувшись с проблемой реализации Программы борьбы с ВИЧ/СПИДом из-за необходимости производить существенно более высокие затраты, правительство Бразилии в 2001 г. приступило к переговорам с ведущими фармацевтическими компаниями. Под угрозой принудительного лицензирования, разрешенного ст. 68-71 Закона 1996 г., правительство смогло эффективно провести переговоры с поставщиками фармацевтической продукции, что привело к значительному снижению в 2001 г. цен на препараты: индинавир (на 64,8 %), эфавиренц (на 59,0%), нелфинавир (на 40,0%) и лопинавир (на 46,0%). Кроме того, между компанией Merck & Coиглавной национальной фармлабораторией Министерства здравоохранения FarManguinhos были заключены соглашения о передаче технологий. В последующие годы переговоры не были так успешны, и зарубежные фармкомпании не шли правительству Бразилии на встречу. Поэтому уже в начале 2007 г. Министерство здравоохранения, проведя еще одни неуспешные переговоры с Merck & Co. Inc., сосредоточенные на цене эфавиренца (efavirenz). В настоящее время эфавиренц - высокоэффективный препарат, используемый 38 %-ми больных ВИЧ/СПИДом в Бразилии, импортировавшийся на тот момент из Индии, где он производился по патенту, распоряжением Правительства от 24 апреля 2007 г. № 886 (Ordinance No. 886) был объявлен препаратом, представляющим национальный общественный интерес (a drug of national public interest)., а уже 4 мая 2007 г. Указом Президента № 6.108 (Decree No. 6.108) на основе необходимости защиты общественного интереса (public interest) в отношении патентов на этот препарат только для некоммерческого использования был установлен режим принудительной лицензии. После этого началось производство этого препарата государственными лабораториями Бразилии. Сразу после этого правительство сообщило о снижении цены на этот препарат, поставляемый из Индии, на 72,2 %. Нужно отметить в этой связи, что, например, в ходе 12 Международного форума «Интеллектуальная собственность – XXI век», прошедшего в Москве 22-26 апреля 2019, в котором автор принимала участие, в одной из секций «Защита интеллектуальных прав в фармацевтическом секторе» обсуждался вопрос целесообразности и эффективности выдачи принудительных лицензий на примере таких стран, как Индия, Канада, США, Германия, Египет и др. [24. С. 42-53] и был сделан вывод о неэффективности этого правового средства.
Представляется, однако, что решение подобных вопросов целесообразно всегда привязывать к конкретным обстоятельствам каждой страны в сфере действия и защиты в ней патентных прав.
Таким образом, можно сделать вывод о том, что действующая политика направлена на достижение существовавшего до вступления в ВТО баланса частных и публичных интересов посредством применения ряда компенсационных механизмов (переговоры о снижении цен с фармкомпаниями, принудительное лицензирование, применение Программы «Народная фармакология Бразилии»). При этом вектор судебных решений, ранее выносимых в пользу частных лиц без учета различных элементов публичной политики в рассматриваемой сфере (напр., избыточность воздействия таких решений на бюджетную сферу, неравномерность доступа к медицине и фармпродуктам среди пациентов и пр.) сменился в сторону большего учета последних, что, как бы то ни было, нельзя признать необоснованным.
References
1. Lei no 5.772, de 21 de dezembro de 1971. Institui o Código da Propriedade Industrial, e do outras providencias. // DOU. de 31.12.1971. URL: http://www.planalto.gov.br/ccivil_03/Leis/L5772.htm (data obrashcheniya: 27.05.2019)
2. Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS). URL: https://www.wto.org/english/docs_e/legal_e/27-trips.pdf (data obrashcheniya: 26.05.2019)
3. Lei 9.279/1996 (Lei Ordinária) 14.05.1996, regula direitos e obrigações relativos a propriedade industrial. // D.O. de 15/05/1996. P. 8353. URL: http://www.planalto.gov.br/ccivil_03/leis/l9279.htm (data obrashcheniya: 10.05.2019)
4. Russell Boltwood. Basic Patent Law in Brazil, and Recent Developments. // International In-house Counsel Journal. Vol. 1, No. 2, January 2008. P. 1-10. URL: https://www.iicj.net/library/detail?key=37 (data obrashcheniya: 26.05.2019)
5. Araújo, Nadia de. 2003. A internalização dos tratados internacionais no direito brasileiro e o caso do TRIPS. Revista da Associação Brasileira da Propriedade Intelectual. São Paulo, no 62, jan-fev, pp.3-14.
6. Basso, Maristela. 2000. O Direito internacional da propriedade intelectual. Porto Alegre: Livraria do Advogado. Tsit. po: Access to Knowledge in Brazil. New research on intellectual property, innovation and development. / Ed. by Lea Shaver. Yale Law School. New Haven. 2007. P. 184.
7. Regional Federal (2ª Região). 2005. Ementa. Patentes de invenção. Acordo TRIPS. Direito à extensão de vigência de patente de 15 para 20 anos. Aplicação do art. 40 da Lei 9.279/96. Documento TRF200139202. Relator: Luiz André Fontes. Órgão julgador: Segunda Turma Esp. 17 de maio. Ibid. P. 210-211.
8. Belikova K.M. Grazhdanskii protsess na strazhe literaturnykh i dr. proizvedenii, soderzhashchikh nauchnuyu informatsiyu, okhranyaemykh avtorskim pravom Brazilii (podkhody stran BRIKS). // Problemy ekonomiki i yuridicheskoi praktiki. – 2019.-№ 3 (v pechati).
9. Belikova K.M. Ugolovno-protsessual'nye aspekty zashchity nauchnoi informatsii, soderzhashcheisya v proizvedeniyakh, zashchishchaemykh avtorskim pravom Brazilii (opyt stran BRIKS). // Probely v rossiiskom zakonodatel'stve. Yuridicheskii zhurnal. – 2019.-№ 3 (v pechati).
10. Constituição da República Federativa do Brasil. // Lei no 13.105/2015 (Código de Processo Civil). Código de Processo Civil e normas correlatas / 9a edição de Senado Federal. Mesa Biênio 2015–2016. Brasília – 2016. P. 16-23. URL: https://www2.senado.leg.br/bdsf/bitstream/handle/id/517855/CPC_9ed_2016.pdf?sequence=3 (data obrashcheniya: 17.05.2019)
11. Meditsina Brazilii. Sistema zdravookhraneniya Brazilii. URL: http://www.russobras.ru/medicine.php (data obrashcheniya: 27.05.2019)
12. Pankov E. Braziliya: nekotorye aspekty sotsial'noi politiki. // Mirovoe i natsional'noe khozyaistvo. – 2014.-№2(29). URL: https://mirec.mgimo.ru/2014-02/brazilia-nekotorye-aspekty-socialnoj-politiki (data obrashcheniya: 27.05.2019).
13. Kurbanov A.R., Lyadova A.V. Zdravookhranenie Brazilii: trudnyi put' k preodoleniyu neravenstva. // Latinskaya Amerika. – 2018.-№ 9. – S. 44-55.
14. Bliss K. Zdravookhranenie kak kompleksnyi politicheskii prioritet. Brazil'skii podkhod k probleme global'nogo zdravookhraneniya v ramkakh vneshnei politiki i initsiativ sodeistviya mezhdunarodnomu razvitiyu. // Vestnik mezhdunarodnykh organizatsii.-2011.-№ 3(34). – S. 83-95.
15. Monica Steffen Guise Rosina, Daniel Wang, Thana Cristina de Campos. Access to medicines: pharmaceutical patents and the right to health. // Access to Knowledge in Brazil. New research on intellectual property, innovation and development. / Ed. by Lea Shaver. Yale Law School. New Haven. 2007. P. 165-197.
16. Luciana Dias de Lima, Marilia Sá Carvalho, Cláudia Medina Coeli. Sistema Único de Saúde: 30 anos de avanços e desafios. Editorial (Escolha das Editoras). // Cad. Saúde Pública 2018; 34(7):e00117118. DOI: 10.1590/0102-311X00117118. URL: http://www.scielo.br/pdf/csp/v34n7/1678-4464-csp-34-07-e00117118.pdf (data obrashcheniya: 27.05.2019)
17. Terrazas, Fernanda. O poder judiciário como voz institucional dos excluídos do processo político: análise do perfil dos beneficiados por decisões judiciais que ordenam o fornecimento de medicamentos. São Paulo: Universidade de São Paulo. 2008. P. 40-43.
18. Belikova K.M. Zashchita prav intellektual'noi sobstvennosti v ES: doktrina ischerpaniya prav i okhrana konkurentnoi sredy. // Intellektual'naya sobstvennost'. Promyshlennaya sobstvennost'. – 2010.-№ 10. – S. 36-44.
19. Bills to amend the Brazilian Patent Statute, Federal Law 9,279 of May 14th, 1996. All the bills included in the timeline seek to amend the patent statute. This timeline does not include bills removed by their sponsors or already approved and signed into laws. Current as of March 2, 2015. / Ed. by Licks Attorneys. URL: http://lickslegal.com/pdf/Timeline_ReformLaw9279.pdf (data obrashcheniya: 28.05.2019)
20. Amicus curiae brief. URL: https://www.ecchr.eu/en/glossary/amicus-curiae-brief/ (data obrashcheniya: 28.05.2019)
21. Lia Hasenclever, Rodrigo Lopes, Gabriela Costa Chaves, Renata Reis. // Revista de Direito Sanitário, São Paulo v. 11, n. 2. P. 164-188. Jul./Out. 2010. URL: https://www.researchgate.net/publication/276393669_O_instituto_de_ patentes_Pipeline_e_o_acesso_a_medicamentos_aspectos_economicos_e_juridicos_deleterios_a_economia_da_saude (data obrashcheniya: 28.05.2019)
22. Ministério da Saúde. Farmácia Popular. 2007. http://portal.saude.gov.br/portal/saude/visualizar_texto.cfm?idtxt=21124&janela=1. Tsit. po: Access to Knowledge in Brazil. New research on intellectual property, innovation and development. / Ed. by Lea Shaver. Yale Law School. New Haven. 2007. P. 198.
23. Rondineli Mendes da Silva, Rosangela Caetano. Programa "Farmácia Popular do Brasil": caracterização e evolução entre 2004-2012. Ciênc. saúde coletiva vol.20 no.10 Rio de Janeiro Oct. 2015. http://dx.doi.org/10.1590/1413-812320152010.17352014. URL: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1413-81232015001002943 (30.05.2019)
24. Vorozhevich A.S. Riski i vozmozhnye posledstviya ogranichenii patentnykh prav v farmsfere. // Vestnik Universiteta imeni O.E. Kutafina (MGYuA). – 2017.-№ 6. – S. 42-53.
25. Jae Sundaram. Pharmaceutical Patent Protection and World Trade Law: The Unresolved Problem of Access to Medicines. Routledge, 1st Edition. 2018. – 240 p. URL: https://www.routledge.com/Pharmaceutical-Patent-Protection-and-World-Trade-Law-The-Unresolved-Problem/Sundaram/p/book/9781138288768 (data obrashcheniya: 17.05.2019
Link to this article
You can simply select and copy link from below text field.
|
|



